|
|
||||||||||||||||||
|
COMPANY
INFO
| MAP Last
Updated: 9/22/2005 • PRICES, SPECIFICATIONS, AND FEATURES SUBJECT TO CHANGE WITHOUT NOTICE.
|
Rainwater is slightly acidic. In heavily industrialised regions, emissions make the rainwater more acidic. The hardness in water comes from the calcium and magnesium salts, that are dissolved into the water, from soluble rocks through which the rain water flows.
Temporary Hardness in most cases is associated with calcium and magnesium carbonates and bicarbonates. These crystal forming salts are held in solution and will remain so, unless there is a change in pressure or temperature, which will cause the water to become supersaturated, resulting in the precipitation of encrusting scale on hot or rough surfaces, such as pipes and heat exchangers. Permanent Hardness is due mainly to calcium and magnesium sulphate and is not effected by heat or pressure change. However if the water is evaporated it will remain and will encrust. The water hardness problem is sometimes exacerbated by being stored in reservoirs constructed from different materials and is also further exacerbated seasonally as the water table rises and falls resulting in concentrations.
Chemists are concerned with the chemical reaction of elements and compounds that have formed as a result of the reactions. To gain an understanding of Physical Conditioners, one has to take into account the physical effects that occur before the reactions take place. Hence Physical Conditioning.
Stable chemical compounds are normally electrically neutral. When they dissolve in water to form solution, they may separate into oppositely charged particles called ions. This process is known as dissociation and can be partial or complete. Although ions are independent particles, the connection to their opposite is maintained and is re-established following crystallisation. This process of dissociation in water is used widely in industry to separate metals from their compounds, for electroplating and the separating of the elements of water itself, Oxygen and Hydrogen gasses.
The mineral salts found in water can be determined in type and quantity by simple evaporation and weighing the residue. In addition to hardness salts - and by other techniques - sodium chloride, sodium sulphate and silica can be found. These substances do not exist in solution as definite compounds, but as "ions" - charged soluble particles of metal (known as cations) or as acid radicals (know as anions). The most commonly occurring cations are:
The most commonly occurring anions are:
The negative and positive signs indicate polarity of electron charge. The negative sign indicates electron gain, positive sign electron loss. Contaminants can be grouped according to polarity and magnitude of charge.
Pure water in its liquid state is also slightly dissociated into its constituent ions. H2O -------> H+ + OH- hydrogen ion, hydroxyl ion This equation suggests that water contain hydrogen ions moving freely within the liquid however a hydrogen atom with one electron removed is simply a proton. It is now recognised that the protons attach themselves to water molecules to form a hydronium ion H3O+. For simplicity, H+ ions will be referred to below, although the physical reality is, that such species do not have an independent existence in water. Both hydrogen and hydroxyl ions are present in exactly the same quantity, so that pure water is "neutral". In a unit weight of pure water there will be 0.0000001 unit weights of Hydrogen ion and of Hydroxyl iron, or 10-7 parts of each. The pH value - the index of acidity, alkalinity or purity - uses the figure 7 as a neutral or purity point of the scale. Natural pure water is said to have a pH value of 7. pH = - log 10 (H+) (where H+ is the hydrogen ion concentration). As hydrogen ion concentration is increased the pH value decreases. As Hydroxyl concentration increases the pH value increases. Acidity is due to hydrogen ions so the more acid the water becomes the lower its pH value Alkalinity is due to hydroxyl ions so the more alkaline the water becomes, the higher its pH value. This is because acids give hydrogen ions in solution, while alkalines give hydroxyl ions: H2SO4 ------> 2H+ + SO42- sulphuric acid NaOH ------> Na+ + OH- caustic soda The pH scale covers the range of 0-14, from strongly acid to strongly alkaline.
Crystallisation normally occurs when a solution becomes supersaturated. A supersaturated solution is one that contains a higher concentration of solute than its equilibrium concentration (saturation). However supersaturation alone is not sufficient for a system to begin to crystallise. It is generally accepted that two steps are involved in the formation of microscopic crystals from supersaturated solutions: First, nuclei, minute crystalline entities of definite size, must be formed (nucleation); and second, these nuclei must grow (crystal growth). There are many other variables that influence the nucleation and growth of crystals such as the presence of the impurities; turbulence within the system; the nature and state of the surfaces in contact with the solution etc. There are two basic nucleation mechanisms:
HydroFLOW produces an electric field which is switched on and off. The length of the off sequence is randomly controlled. By switching the electric field off, adjacent species move together to form clusters (see above). These clusters, themselves representing larger dipoles, are affected by the electric field during the on sequence, and are joined together to form localised areas of high concentration. The internal forces generated by these larger clusters result in a contraction and concentration of the attraction forces, this causes the collapse of the clusters into nuclei, which are the seed crystal.
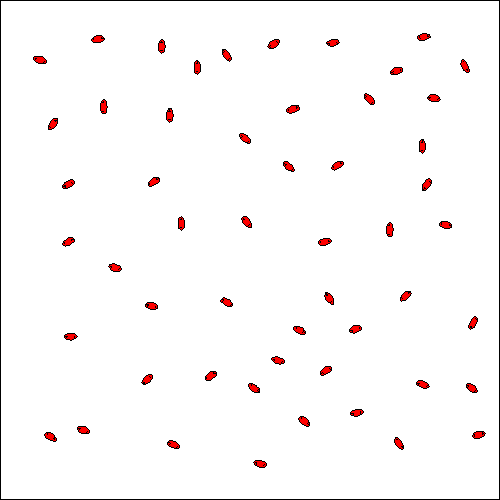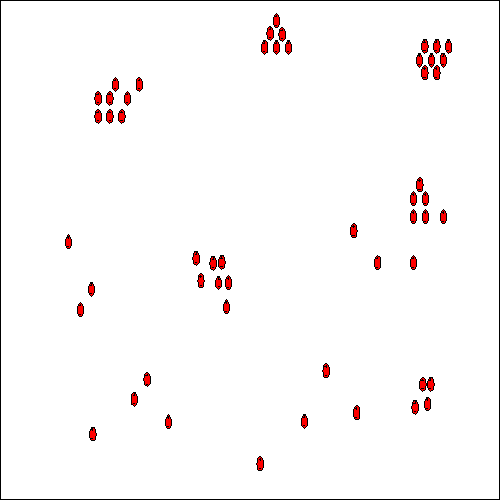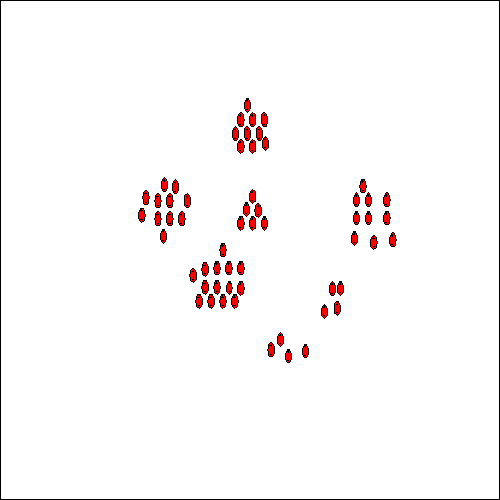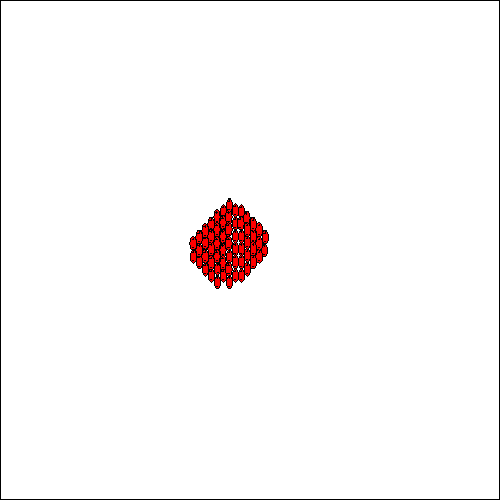
The presence of the HydroFLOW electric field throughout the solute will enhance the formation of these large clusters by orientating them, in both the saturated and unsaturated solutions. This process attracts more charged species and stable nuclei are formed (see Fig. 6). The attraction forces of such nuclei become much greater and as ions diffuse to the nuclei surface, a diffusion layer is formed and the ions become incorporated into the crystal lattice. Crystals are formed and grow, again helped by the effect of orientation of the ions by the applied field, and aggregate to form larger crystals. (When HydroFLOW is used, the diffusion is enhanced because the ions are orientated by the electric field being applied.) For this process to occur throughout the system, the field has to be present throughout the solution, especially closer to the area where the solution will experience changes in temperature or pressure which are responsible for the precipitation of the salts from solution. When HydroFLOW is present, crystals are formed and grow helped by the orientation of the ions in the applied field. Small crystals aggregate to form larger crystals that grow at the expense of smaller crystals.
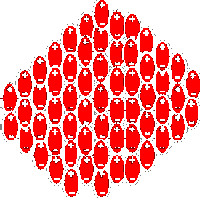
If hard scale deposits are to be avoided, heterogeneous nucleation has to be minimised and homogeneous nucleation under supersaturation conditions has to be prevented. This can be achieved by the installation of HydroFLOW that will start the generation of large numbers of nuclear clusters. These clusters will grow and then collapse into nuclei that will act as seed crystals. In the presence of a large quantity of seeds, homogeneous crystallisation can occur in the solution. This will cause the formation of large crystals as soon as the solution approaches super-saturation. Large crystals then grow at the expense of smaller crystals. The bulk of crystallisation will occur in suspension, and heterogeneous crystallisation on the surfaces is thereby minimised. Any heterogeneous crystallisation that does form on the surfaces, will be such a thin layer that it will be returned to solution, as soon as the solution becomes unsaturated.
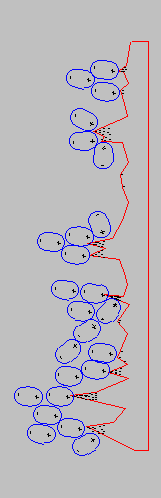
In a scaling system there are three processes that are at work: Heterogeneous crystallisation, homogeneous crystallisation, and the return to return to solution of the scale when the solute has become unsaturated. Heterogeneous crystallisation occurs primarily, on surfaces that are subject to increasing temperatures. As not all the solute is in contact with the heating surface, convection and circulating currents will carry supersaturated liquid away to other surfaces. Scaling on other surfaces will continue until saturation point is achieved. Homogeneous crystallisation occurs in large vessels containing high volumes of solute, with a relatively small surface area. As the solute is being heated, the solution becomes supersaturated. The surface area is not sufficient to provide all the nucleation necessary. The solute reaches a critical condition. At this point any source of energy, such as turbulence in the solute, will cause homogeneous nucleation. All the material that can precipitate does so at once. A large number of small crystals are formed. These crystals have high surface charges that cause them to adhere to all the surfaces, including cold surfaces. The fine crystals that have adhered to the surfaces will then become the nuclei for heterogeneous crystallisation in subsequent heating cycles. The third process is the return to solution of the scale deposits. After the solute has become unsaturated due to cooling or pressure change, a quantity of the deposits will be returned to solution. The surface scale that had been formed is not as stable as the crystals that have been formed in suspension, due to the uneven way that nucleation has occurred on the surfaces (see Fig. 7). Descaling can only occur if the water in contact with the scaled surface is unsaturated, and is able to dissolve the carbonates to form bicarbonates. The presence of CO2 is necessary for the formation of bicarbonates. The CO2 which is present in solution in the water, comes from two sources, one from the air in contact with the water and the other, from the decomposition of bicarbonates due to the heating process. CaCO3 + H2O +CO2---------> Ca (HCO3)2 unsaturated water Descaling of the heat exchanger using HydroFLOW relies totally on turbulence. This is because the temperature of the water is increasing and would normally only deposit scale. If turbulence is present, the water experiences pressure changes that cause the water to change rapidly from supersaturated to an unsaturated condition. While unsaturated the water will dissolve the scale on the surfaces, and in the supersaturated condition the deposits will grow in suspension due to the presence of the clusters generated by the HydroFLOW applied field. In every system containing solute, there is a balance of scale-formation and scale-solution. In a system where the balance is in favour of scale-formation, the system will experience scaling. In a system where the balance is in favour of scale-solution, the system will remain free of scale. Ca (HCO3)2 + Heat + HydroFLOW ------> CAC3 + H2O + CO2 calcium bicarbonate, calcium carbonate, in solution in suspension CaCO3 + turbulence + HydroFLOW + H2O + CO2---------> Ca (HCO3)2 calcium carbonate, calcium bicarbonate, on the surfaces HydroFLOW simply tips the balance in favour of the scale-solution, by providing a large quantity of unsaturated solution that dissolves the existing surface scale. This process is repeated, dissolving surface scale and forming suspended stable crystals. The heterogeneous crystallisation is replaced by homogeneous crystallisation. However in this case homogeneous crystallisation occurs as soon as the solute becomes supersaturated, due to the presence of a large quantity of clusters generated by HydroFLOW. As a result the old scale will ultimately completely return to solution and is converted to stable individual crystals. These stable amorphous crystals can be removed by filtration in circulating systems. In open systems they will pass harmlessly out with the flow |
||||||||||||||||||||||||||||||||||||||||||||||||||